The bioMD+ Collection:
The bioMD+ Collection:
131 Reviews
From: $95.00 — or from From: $95.00$42.75 every 28 days
60 Reviews
$140.00 — or $140.00$63.00 every 28 days
91 Reviews
$125.00 — or $125.00$50.00 every 28 days
180 Reviews
From: $75.00 — or from From: $75.00$33.75 every 28 days
180 Reviews
From: $75.00 — or from From: $75.00$33.75 every 28 days
170 Reviews
From: $75.00 — or from From: $75.00$33.75 every 28 days
131 Reviews
From: $95.00 — or from From: $95.00$42.75 every 28 days
Quality is our top priority
bioMD+ strives to offer high quality plus CBD products that people can trust.
Our products contain only the best 100% Organic Colorado hemp. Grown and specially formulated with our customers’ wellness in mind, we provide accessible options for everyone. So whether you’re a returning customer our you’re just getting started, we’ll be here to provide the best CBD products.
Quality is our top priority
bioMD+ strives to offer high quality CBD products that people can trust.
Our products contain only the highest quality 100% Organic Colorado hemp. Grown and specially formulated with our customers’ wellness in mind, we provide accessible options for everyone. So, whether you are already part of the bioMD+ family or you are just getting started in CBD, we will be here to provide you with the BEST CBD products available.

Why bioMD+
Our products are produced using innovative technology with a mission to provide fast, strong, and long-term relief. In addition to highly effective and unique product formulations, our products are guaranteed to be gluten-free, non-GMO, vegan, and 3rd party tested! We go the extra mile to create extraordinary products. So that you can have peace of mind knowing that you are truly getting the best products from the most trusted and reliable brand. The bioMD+ promise is to always prioritize people over profit.



bioMD+ CBD Tinctures
bioMD+ offers a new line of the best CBD tinctures and top-quality CBD Gummies that combine advanced science with the finest organic and natural ingredients.
bioMD+ CBD Tinctures
bioMD+™ offers a new line of products that combine advanced science with the finest organic and natural ingredients, engineered to deliver powerful relief and relaxation.
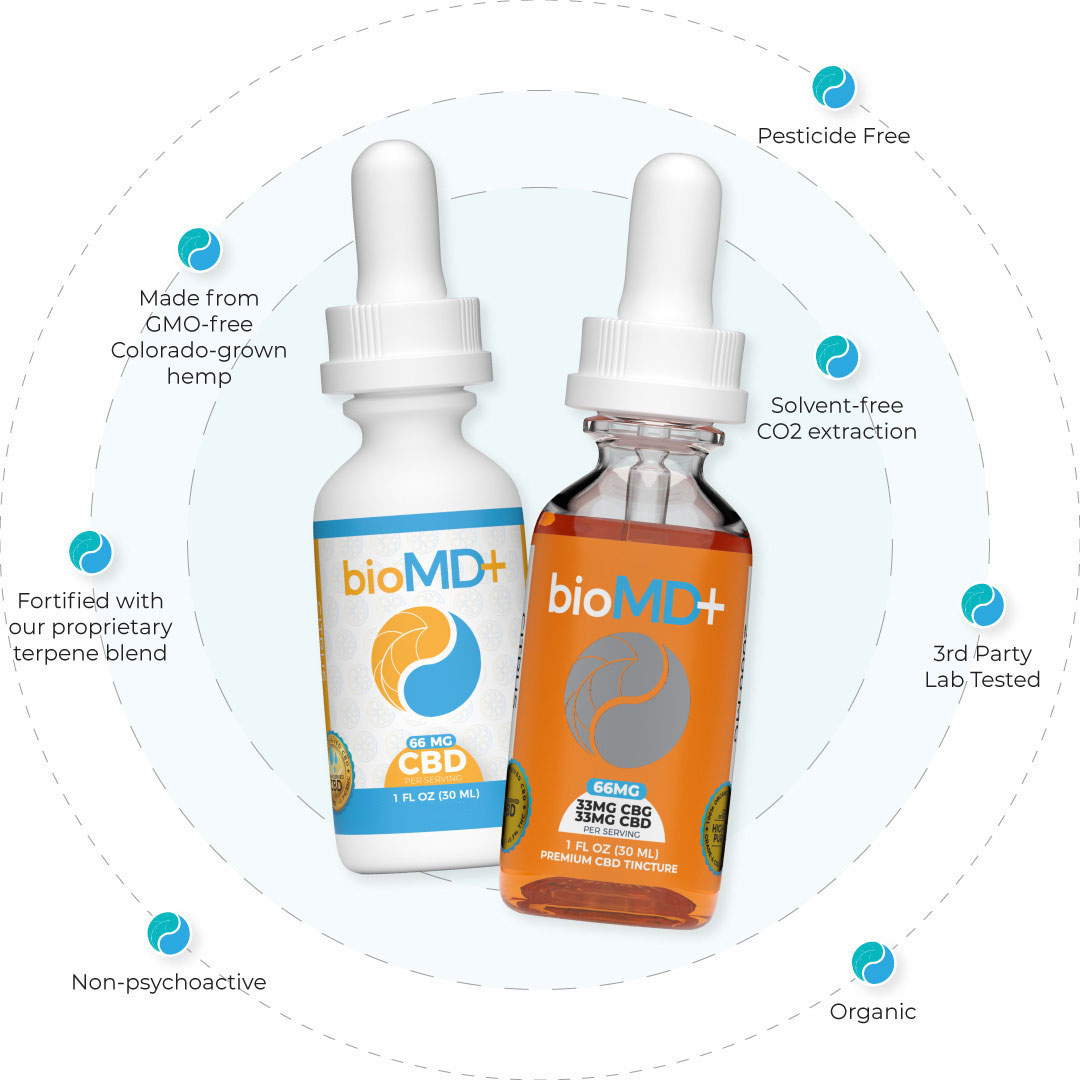
Why Buy Our CBD Products?
bioMD+ provides most effective CBD oil products on the market today. We provide you with the best quality CBD products possible, which can be seen in the bio MD plus CBD oil reviews and third-party lab reports.
Why bioMD+
Our products are produced using innovative technology with a mission to provide fast, strong, and long-term relief. In addition to highly effective and unique product formulations, our products are guaranteed to be gluten-free, non-GMO, vegan, and 3rd party tested! We go the extra mile to create extraordinary products. So that you can have peace of mind knowing that you are truly getting the best products from the most trusted and reliable brand. The bioMD+ promise is to always prioritize people over profit.



What is CBD?
CBD is an acronym for Cannabidiol (Can-a-bi-di-ol), a prominent naturally occurring class of molecules called cannabinoids found in the plant species Cannabis sativa. CBD comprises up to 40% of the plant and is one of over 60 plus compounds found in cannabis. Of these compounds, CBD and THC are usually present in the highest concentrations and are therefore the most recognized and studied.
Subscribe to our newsletter
Stay up to date on the latest updates and discounts.
Want a free gift? We send our CBD every month as a giveaway! Join our newsletter and you might win our giveaway!